 Pharmaceutical Waste must be disposed of as Chemical Waste through EH&S
Pharmaceutical Waste must be disposed of as Chemical Waste through EH&S
Many chemical and/or pharmaceutical compounds used in research or in the treatment of disease at the University of Florida are regulated by the EPA as listed or characteristic hazardous wastes when disposed. Many other pharmaceutical compounds are not regulated as hazardous waste but may pose a threat to human health or the environment and have the potential to be misused. All unusable prescription or non-prescription pharmaceuticals and pharmaceutical compounds used in treatment or research should be disposed of through EH&S as Hazardous Waste.
A limited number of prescription pharmaceuticals and pharmaceutical compounds used in research and treatment are regulated by the Drug Enforcement Administration under the Controlled Substances Act. EH&S does not collect controlled substances for disposal.
Hazardous Waste Pharmaceuticals
Many chemical and/or pharmaceutical compounds used in research or in the treatment of disease at the University of Florida are regulated by the EPA as Listed or Characteristic Hazardous Wastes when disposed. The following lists are examples of some common Hazardous Waste Pharmaceuticals.
P-Listed Hazardous Waste Pharmaceuticals
Unused dilutions, formulations (including container rinsates) or wastes generated through spillage of the original product are considered hazardous waste. Packaging and/or empty containers associated with these items must either be collected as hazardous waste or triple-rinsed before being considered empty.
| P012 |
Arsenic Trioxide |
|
P081 |
Nitroglycerin |
| P042 |
Epinephrine |
|
P188 |
Physostigmine salicylate |
| P046 |
Phentermine |
|
P204 |
Physostygmine |
| P075 |
Nicotine and Salts |
|
|
|
U-Listed Hazardous Waste Pharmaceuticals
Unused dilutions or formulations or wastes generated through spillage of the original product are considered hazardous waste. Personal Protective Equipment PPE contaminated during use or preparation of Chemotherapy agents should be collected as hazardous waste.
| U010 |
Mytomycin C |
U058 |
Cyclophosphamide |
| U035 |
Chlorambucil |
U059 |
Daunomycin |
| U052 |
Cresol |
U055 |
Cumene |
Characteristic Hazardous Waste Pharmaceuticals
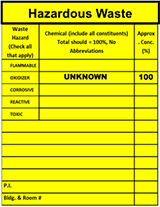
Pharmaceutical chemicals or formulations may also contain components which are regulated as characteristic waste. The presence of these components is often present only as a component (often a minor constituent) and not clearly identified in labeling. The EPA regulates Toxicity Characteristic chemicals at the parts per million (ppm) level. Knowledge and understanding of the entirety of product make-up is critical for safety and proper waste determination.
| Thimerosol, Merthiolate |
Toxic (Mercury) |
|
Silvadene |
Toxic (Silver) |
|
| Barium Sulfate |
Toxic (Barium) |
|
Insulin |
Toxic (m-Cresol) |
|
| Mercurochrome |
Toxic (Mercury) |
|
Styptic Pens |
Toxic (Silver) |
|
|
|
|
Selenium Sulfide |
Toxic (Selenium) |
|
|
|
|
|
|
|
Prescription and Non-Prescription Pharmaceuticals
Pharmaceutical Waste must be disposed of as Chemical Waste through EH&S
Prescription and “over the counter,” non-prescription pharmaceutical waste generated while in use at the Student Infirmary or Veterinary Hospital should be disposed of as Hazardous Waste through EH&S. Prescription Pharmaceutical wastes also include solutions and formulations not in the original packaging (IV bags, etc). Pharmaceutical waste does NOT include Sharps or other material considered to be Medical or Bio-Hazard Waste (IV sets, syringes, needles, etc). Unopened pharmaceuticals should be returned for credit through a pharmaceutical reverse distributor. UF is not responsible for the disposal of unused personal or pet prescriptions. Personal prescriptions may be disposed of through the Alachua County Disposal of Unwanted Medicines program.
Disposal of Controlled substances
The Drug Enforcement Agency’s (DEA) Office of Diversion Control regulates the disposal of DEA controlled substances.
Proper use and disposal of DEA controlled substances is the responsibility of the individual holding the DEA registration used to obtain them. Maintaining accurate and current records of usage and maintaining a current DEA registration are critical for compliance with DEA regulations. Principle Investigators who “orphan” controlled substances by abandoning or losing control of them are in violation of federal law. Do not create orphan drugs. If drugs are found, contact Chemical and Radioactive Waste Disposal for assistance at (352) 392-8400.
Reverse Distribution
All reverse distributors have their own slightly different methods and requirements. The process typically involves most if not all of the following:
- Contact a Reverse Distribution Vendor to register.
- Complete the vendor’s application for approval to ship material – either in paper form and sent by mail / fax or in electronic form (both require current DEA registration).
- Additional Forms – Completing DEA D-222 form if necessary (for Schedule I & II controlled substances)
- Payment – payment or payment information is required with application.
- Shipping – once authorized, items are packaged and shipped by you via an approved shipper (Fed-Ex or UPS usually)
- Always request documentation of return/disposal/destruction
Reverse Distributor Vendor List
The following is a list of DEA registered reverse distributors licensed to provide return/disposal services in the State of Florida:
PharmaLink (800) 257-3527
Rx Reverse Distributors, Inc. (866) 388-7973
Triumverate, Inc. (888) 834-9697
Other Resources






 Prior to ordering pressurized gas cylinders for their research projects, Principal Investigators and lab members are directed to contact the manufacturer or cylinder supplier and make arrangements to have the cylinders picked up following use.
Prior to ordering pressurized gas cylinders for their research projects, Principal Investigators and lab members are directed to contact the manufacturer or cylinder supplier and make arrangements to have the cylinders picked up following use. Aerosol cans which are empty of all contents are allowed to be disposed of as “regular” trash by placing in any waste receptacle. If there are contents still in the can, however, the aerosol product should be placed in an appropriate outer container (such as a fiberboard drum) in the lab’s Hazardous Waste Satellite Accumulation Area and disposed of through EH&S.
Aerosol cans which are empty of all contents are allowed to be disposed of as “regular” trash by placing in any waste receptacle. If there are contents still in the can, however, the aerosol product should be placed in an appropriate outer container (such as a fiberboard drum) in the lab’s Hazardous Waste Satellite Accumulation Area and disposed of through EH&S. Dinitro- and trinitro- compounds: should be disposed of through EH&S as hazardous waste once the compound is noted to be undergoing physical change. If the product is past its printed expiration date or is exhibiting signs of drying or crystallization. It should be disposed of through EH&S. Contact EH&S if you discover very old containers or containers with metallic lids.
Dinitro- and trinitro- compounds: should be disposed of through EH&S as hazardous waste once the compound is noted to be undergoing physical change. If the product is past its printed expiration date or is exhibiting signs of drying or crystallization. It should be disposed of through EH&S. Contact EH&S if you discover very old containers or containers with metallic lids. Peroxide Forming Chemicals: many common organic chemicals and some common inorganic chemicals can form potentially explosive peroxides during storage following exposure to atmospheric oxygen and UV radiation. To prevent peroxide formation and to promote disposal of potentially unstable chemicals, items listed in the table below should be stored in tightly closed containers, away from light and disposed of after their useful lifespan. To track the lifespan, these chemicals should be dated upon receipt and dated upon first opening.
Peroxide Forming Chemicals: many common organic chemicals and some common inorganic chemicals can form potentially explosive peroxides during storage following exposure to atmospheric oxygen and UV radiation. To prevent peroxide formation and to promote disposal of potentially unstable chemicals, items listed in the table below should be stored in tightly closed containers, away from light and disposed of after their useful lifespan. To track the lifespan, these chemicals should be dated upon receipt and dated upon first opening.
 High Performance Liquid Chromatography (HPLC) equipment often creates hazardous liquid waste. The process frequently results in wastes which contain flammable solvents such as acetonitrile or methanol, and these are often mixed with acidic components such as formic or trifluoroacetic acid.
High Performance Liquid Chromatography (HPLC) equipment often creates hazardous liquid waste. The process frequently results in wastes which contain flammable solvents such as acetonitrile or methanol, and these are often mixed with acidic components such as formic or trifluoroacetic acid.
 The hazardous effluent from the HPLC equipment must be collected in closed containers. Labs may purchase purpose-built collection caps/containers for this; an easy solution, however, is to simply drill holes through the cap of any suitable container and route the effluent lines through those ports.
The hazardous effluent from the HPLC equipment must be collected in closed containers. Labs may purchase purpose-built collection caps/containers for this; an easy solution, however, is to simply drill holes through the cap of any suitable container and route the effluent lines through those ports.